CAMKK2
calcium/calmodulin dependent protein kinase kinase 2




These figures show a summary of data collected by the cancer genome atlas for CAMKK2. The mutations heatmaps shows the fraction samples with each type of genetic mutation, while the copy number variation shows the percentage of samples where a deletion or amplication was dectected. Finally, the mRNA expression tab shows the amount of mRNA detected on a log-2 scale for each cancer type. The X-axis cancer type abbreviations are described here. This summary of the cancer genome atlas (TCGA) was collated from firebrowse developed by the Broad Institute. The code used to produce these figures is available through github.
Kinase Domain Structure:
- Title: Crystal structure of human calcium/calmodulin-dependent protein kinase kinase 2, beta, CaMKK2 kinase domain in complex with STO-609
- Resolution: 2.4
Associated Compounds:
- 7-oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: Crystal Structure of the Human CAMKK2B bound to GSK650394
- Resolution: 2
Associated Compounds:
- 2-cyclopentyl-4-(5-phenyl-1H-pyrrolo[2,3-b]pyridin-3-yl)benzoic acid
- FORMIC ACID
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: Crystal Structure of the Human CAMKK2B in complex with TAE-226
- Resolution: 2
Associated Compounds:
- ACETATE ION
- 2-({5-CHLORO-2-[(2-METHOXY-4-MORPHOLIN-4-YLPHENYL)AMINO]PYRIMIDIN-4-YL}AMINO)-N-METHYLBENZAMIDE
- CHLORIDE ION
- 1,2-ETHANEDIOL
- AMMONIUM ION
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: Human CAMKK2 with GSK650393
- Resolution: 2.4
Associated Compounds:
- 1,2-ETHANEDIOL
- 2-(2-methylpropyl)-4-(5-phenyl-1H-pyrrolo[2,3-b]pyridin-3-yl)benzoic acid
- FORMIC ACID
- PHOSPHOSERINE
- PHOSPHOTHREONINE
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: Crystal Structure of the Human CAMKK2B in complex with CP673451
- Resolution: 1.9
Associated Compounds:
- 1-{2-[5-(2-methoxyethoxy)-1H-benzimidazol-1-yl]quinolin-8-yl}piperidin-4-amine
- GLYCEROL
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: Crystal Structure of the Human CAMKK2B in complex with BI2526
- Resolution: 1.8
Associated Compounds:
- ACETATE ION
- 4-{[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,7,8-tetrahydropteridin-2-yl]amino}-3-methoxy-N-(1-methylpiperidin-4-yl)benzamide
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: Crystal Structure of the Human CAMKK2B
- Resolution: 1.7
Associated Compounds:
- 2-cyclopentyl-4-(5-phenylfuro[2,3-b]pyridin-3-yl)benzoic acid
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: A kinase-inhibitor complex
- Resolution: 2.57
Associated Compounds:
- 3-(1H-tetrazol-5-yl)-10lambda~6~-thioxanthene-9,10,10-trione
- S-(DIMETHYLARSENIC)CYSTEINE
- CHLORIDE ION
- GLYCEROL
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: A kinase-inhibitor complex
- Resolution: 2.02
Associated Compounds:
- (3Z)-5-chloro-3-[(1-methyl-1H-pyrazol-4-yl)methylidene]-1,3-dihydro-2H-indol-2-one
- S-(DIMETHYLARSENIC)CYSTEINE
- CHLORIDE ION
- GLYCEROL
- TRIETHYLENE GLYCOL
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: A kinase-inhibitor complex
- Resolution: 2.02
Associated Compounds:
- S-(DIMETHYLARSENIC)CYSTEINE
- CHLORIDE ION
- GLYCEROL
- TRIETHYLENE GLYCOL
- 3-{2,4-dimethyl-5-[(Z)-(2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-1H-pyrrol-3-yl}propanoic acid
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: A kinase-inhibitor complex
- Resolution: 1.93
Associated Compounds:
- 1-amino-4-hydroxy-9,10-dioxo-9,10-dihydroanthracene-2-carboxylic acid
- S-(DIMETHYLARSENIC)CYSTEINE
- CHLORIDE ION
- GLYCEROL
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: A kinase-inhibitor complex
- Resolution: 2.53
Associated Compounds:
- 5-chloro-2-methoxy-4[(1Z)-3-(4-methoxyphenyl)-3-oxoprop-1-en-1-yl]aminobenzoic acid
- S-(DIMETHYLARSENIC)CYSTEINE
- CHLORIDE ION
- GLYCEROL
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: Crystal Structure of the Human CAMKK2B in complex with Crenolanib
- Resolution: 1.95
Associated Compounds:
- 1-(2-{5-[(3-Methyloxetan-3-yl)methoxy]-1H-benzimidazol-1-yl}quinolin-8-yl)piperidin-4-amine
- 1,2-ETHANEDIOL
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: Crystal Structure of the Human CAMKK2B bound to a thiadiazinone benzamide inhibitor
- Resolution: 1.9
Associated Compounds:
- 4-({5-[(3-hydroxy-4-methylphenyl)amino]-4-oxo-4H-1,2,6-thiadiazin-3-yl}amino)benzamide
- MAGNESIUM ION
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: Crystal Structure of the Human CAMKK2B in complex with AP26113-analog (ALK-IN-1)
- Resolution: 2.2
Associated Compounds:
- 5-chloro-N~2~-{4-[4-(dimethylamino)piperidin-1-yl]-2-methoxyphenyl}-N~4~-[2-(dimethylphosphoryl)phenyl]pyrimidine-2,4-diamine
- SODIUM ION
View This Structure on RCSB PDB
Kinase Domain Structure:
- Title: Crystal Structure of the Human CAMKK2B
- Resolution: 1.6
Associated Compounds:
- 2-cyclopentyl-4-(7-methoxyquinolin-4-yl)benzoic acid
View This Structure on RCSB PDB

Compound Name: SGC-CAMKK2-1
Chemical Name: 2-cyclopentyl-4-(5-(m-tolyl)furo[2,3-b]pyridin-3-yl)benzoic acid
CHEBI: 156444
Smile String: CC1=CC=CC(C2=CC(C(C3=CC(C4CCCC4)=C(C(O)=O)C=C3)=CO5)=C5N=C2)=C1
Chemical Formula: C26H23NO3
Molecular Weight: 397.5
cLogP: 5.53
Source: SGC-UNC
This compound is available through special request from the SGC-UNC.
Additional data concerning this compound can be found here.

This data stems from the differentiation of neural stem cells into fully functional neurons and glia, which requires precise regulation of diverse molecular pathways over time and space. As part of the Harvard Medical School Library of Integrated Network-based Cellular Signatures (LINCS) Program (NIH grant U54 HL127365, lincs.hms.harvard.edu), we used phospho-proteomics to assess changes during ReN VM cell differentiation. Depicted is the dark kinase of interest (black) and two reference kinases (blue and green) to aid interpretation of the values. More information on this work can be found on Synapse.
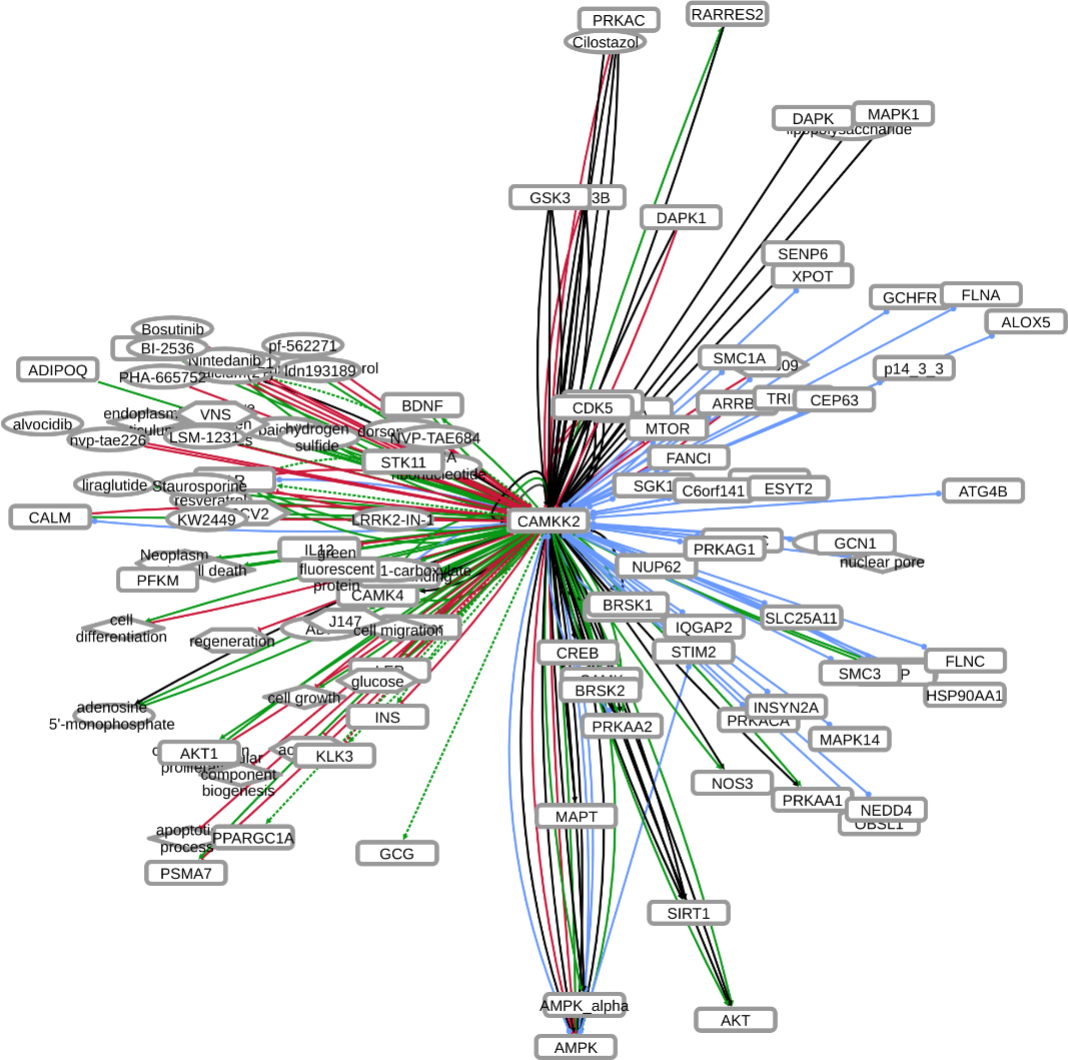
INDRA (Integrated Network and Dynamical Reasoning Assembler) is an automated model assembly system drawing from natural language processing systems and structured databases. It collects mechanistic and causal assertions, represents them in a standardized form (INDRA Statements), and assembles them into various modeling formalisms including causal graphs and dynamical models. More information on this work can be found on Github. In this particular figure, several interaction-types are depicted; physical complexes (blue), phosphorylation (black), and general up- or downregulation (green and red, respectively). Biomacromolecules are represented as squares, small molecule as circles, and biological processes and diamonds. The thickness of each line reflects a confidence score, with thicker lines higher in confidence.
Affinity Purification - Mass Spectrometry
The expression of kinases varies widely across the human tissues assayed by the GTEx project and the Human Proteome Map. To gain a better understanding of the kinase tissue distribution, we've created an application that describes and summarizes the expression of each dark kinase in the context of the rest of the kinome. This interactive window onto the full applications shows the data associated with CAMKK2. The graph summarizes the expression of the kinase across all the organ systems.
The full application can further explored at the Kinase Expression Data Application.
The Reactome Knowledgebase of Human Biological Pathways and Processes is a curated and peer-reviewed knowledgebase available online as an open access resource that can be freely used and distributed by all members of the biological research community. This view of the reactome database is focused on CAMKK2 and displays the pathways associated with CAMKK2.